A low-cost method for collecting water from the atmosphere
By: Saurabh Kulkarni | Date: 15th September 2019

It is reassuring to know that, while four billion people are facing water scarcity, the global climate strike of September 2019 gathered more than four million people of all ages to help the elevate fight against climate change.
It shows awareness and urgency among the people around the globe. But the social will should always go hand in hand with the sustainable technological advancement to curb major issues such as water scarcity and climate change. This article presents one such discovery that can help reduce the water scarcity in an economical, sustainable and efficient way.
Due to absence of water supplies (ground and surface water both) to many parts of the world especially places such as sub-Saharan countries of Africa as well as in our own states such as Maharashtra, Tamil Nadu and others, where the water has to be transported over long distances making it an expensive and inefficient solution.
But even in desert areas the atmosphere is replete with the water vapour equivalent to 10% of water in the lakes and almost 6 times the water in the river on this planet.
Since last decade there have been numerous researches on how to capture atmospheric water. One such recent research was successfully carried out by Ph.D. student Renyuan Li and his supervisor Peng Wang from KAUST in 2018 by testing the commonly available salt used at night to extract water vapour and then releasing the drinking water when salts are exposed to sunlight.
 Image Source: kaust.edu.sa
Image Source: kaust.edu.sa
Above researchers tested the physical and chemical stability of 14 common anhydrous and hydrated salt couples and also tested their water-vapour capturing capacities. They found out 3 salts to be highly effective as seen in fig.2 namely copper chloride (CuCl2), copper sulphate (CuSO4), and magnesium sulphate (MgSO4).
The parameters in which these 3 salts stood out were water-vapour capturing even in as low humidity as 15% and releasing of water even if exposed to not-so-bright sunlight. Li said that sunlight factor would be a huge differentiator as it makes the salts viable to use in the regions where sunlight is very limited and increases the utility range (area-wise) of the salts.
This research shows a good promise in developing a low-cost and efficient method and with far lesser investment but an absorbent- commonly used salts and the release source- sunlight. Dry regions with poor water amenity but a little over 15% humidity and tinge of sunlight can now avail fresh drinking water without hustling too much.
 Image Source: kaust.edu.sa
Image Source: kaust.edu.sa
The phenomenon of capturing and holding water from the atmosphere is called Hygroscopy. Cotton, paper, sugar, caramel, honey, glycerol, ethanol, wood are some of the hygroscopic substances. Some of the salts that are intensely hygroscopic are zinc chloride, calcium chloride, potassium hydroxide and sodium hydroxide.
The relative humidity tells how much quantity of water-vapour can hydroscopic material can hold, i.e. both are proportional. Many a times these materials find application as desiccants (substance used to keep vicinity moisture free, small packet we find in packed electronic devices, sometimes used with rice storage).
Also, Professor Wand and Renyuan Li along with their team at KAUST built a simple and low-cost yet efficient device made from hydrogel (A hydrogel is a three-dimensional (3D) network of hydrophilic polymers that can swell in water and hold a large amount of water while maintaining the structure due to chemical or physical cross-linking of individual polymer chains.) based material that help capture the water from the air and release it for drinking and other purposes using sunlight.
Hydrogel used here is made up of nontoxic salt called calcium chloride which is cheap and stable. As discussed earlier calcium chloride is highly hygroscopic in nature so much so that it in the form of hydrogel absorbs amount of water-vapour that is 6 times its weight.
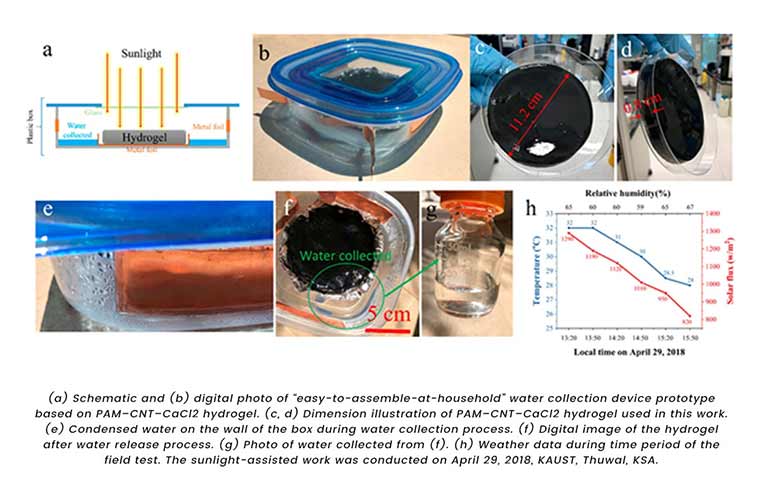 Image Source: acs.org
Image Source: acs.org
While testing the prototype of the device KAUST team used 35 grams of hydrogel and obtained 37 grams of water overnight, with 60% humidity.
Next day the same amount of absorbed water was released and collected from the device after 2.5 hours of exposure to sunlight. “The hydrogel’s most notable aspects are its high performance and low cost,” expressed Li.
“If the prototype were scaled up to produce 3 liters of water per day—the minimum water requirement for an adult—the material cost of the adsorbent hydrogel would be as low as half a cent per day.” Professor Wang said “The next step will be to fine tune the absorbent hydrogel so that it releases harvested water continuously rather than in batches.”
Pulling water from air can be done in mainly two ways- cooling condensation and using desiccants such as zeolites, metal−organic frameworks (MOF), or hygroscopic salts. As the days pass by research in Atmospheric Water Generator is increasing exponentially all over the world.
And the amount of water stress India is steeped into, research and innovation is where our focus and money needs to be invested into. As the start-up culture is on the rise here in India, people can take notice of this issue and find sustainable, low-cost as well as commercial solution out of it, not just concept-wise but also production-wise.
A lot of ground work has already been done by many organisations and universities; we can take their help and keep the wheel of innovation moving forward according to our country’s needs. Times have changed now, salt is not an old fashioned wound burner or a taste maker, it can be a life saver too!